Ceftiofur
 | |
| Clinical data | |
|---|---|
| Trade names | Naxcel |
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
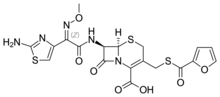
| Formula | C19H17N5O7S3 |
| Molar mass | 523.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ceftiofur is an antibiotic of the cephalosporin type (third generation), licensed for use in veterinary medicine. It was first described in 1987.[1] It is marketed by pharmaceutical company Zoetis as Excenel,[2] Naxcel, and Excede and is also the active ingredient in that company's Spectramast LC (lactating cow formulation) and Spectramast DC (dry cow formulation) product.[citation needed]
It is resistant to the antibiotic resistance enzyme beta-lactamase, and has activity against both Gram-positive and Gram-negative bacteria. E. coli strains resistant to ceftiofur have been reported.[3]
The metabolite desfuroylceftiofur also has antibiotic activity.[4] The two compounds are measured together to measure for antibiotic activity in milk (alongside other antibiotics).[5]
References[edit]
- ^ Yancey RJ, Kinney ML, Roberts BJ, Goodenough KR, Hamel JC, Ford CW (1987). "Ceftiofur sodium, a broad-spectrum cephalosporin: evaluation in vitro and in vivo in mice". Am. J. Vet. Res. 48 (7): 1050–3. PMID 3631686.
- ^ "Pfizer Animal Health Dairy Information on Products and Solutions". Archived from the original on 2007-10-13. Retrieved 2007-11-20.
- ^ Donaldson SC, Straley BA, Hegde NV, Sawant AA, DebRoy C, Jayarao BM (2006). "Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves". Appl. Environ. Microbiol. 72 (6): 3940–8. Bibcode:2006ApEnM..72.3940D. doi:10.1128/AEM.02770-05. PMC 1489609. PMID 16751500.
- ^ Salmon SA, Watts JL, Yancey RJ (July 1996). "In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance". Journal of Veterinary Diagnostic Investigation. 8 (3): 332–336. doi:10.1177/104063879600800309. PMID 8844576.
- ^ "BetaStar Plus / For beta-lactam antibiotics / Product information sheet" (PDF). Neogen. Retrieved 9 September 2014.
